
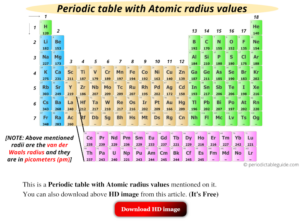
The atomic radius of an electron decreases over a time period when valence electrons are added to the same level of energy while the nucleus increases in protons. What causes the decrease in atomic radius over a period? It is usually the average distance between the nucleus and the boundary of surrounding electron shells. Moreover, the radius of alkali metals is metallic radius and that of halogens is covalent radius and metallic radius > covalent radius. An element located closer to the lower left corner of the periodic table will generally have the larger atomic radius, while an element located closer to the. While in a group atomic radius, increases from up to down. > Which one of the following has the small. > Classification of Elements and Periodicity in Properties. The atomic radius is the measurement of the size of an element chemical. When we move from left to right in the Periodic Table, atomic radius decreases. Which one of the following has the smallest atomic radius Class 11.
#Which atom has the smallest atomic radius how to#
This will tell you how to calculate the radius of an atom's atomic nucleus. As you move down the periodic tree, the number of filled electronshells is increasing. As you go down the group from top to bottom, the ionic radius of each cation increases, just as it does for the radius of each atom because an energy level (. The atomic numbers are larger than the atoms' radius. Second, what is the effect of atomic radius? The atomic number of an element is a function of the size of its nucleus, and the number electrons surrounding it. Yeah, He is even smaller than hydrogen, H, which is 53 pm. The atomic radius can be defined as the ionic radius or covalent radius, metallic radius or van der Waals radius. The smallest atom on the periodic table is helium, He, and has a radius of 31 pm.

This value is not defined in a standard way. Since all the elements given are in the same period (row), the one that is furthest right has the smallest radius, and that would be Br (bromine).

Atomic radius is a term that describes the atom. Which atom has a smaller atomic size as or br Atomic radius of an element increases going from right to left and from top to bottom in the periodic table. While the Earths atmosphere near the mean sea level contains 1019 atoms in a. This term describes the size of an Atoma, but it's not precise D. The smallest ones are A-class, followed by B, C, M and X.


 0 kommentar(er)
0 kommentar(er)
